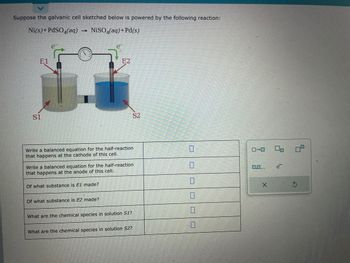
Transcribed Image Text:Suppose the galvanic cell sketched below is powered by the following reaction:
Ni(s) + PdSO4(aq)
1 NiSO4(aq) + Pd(s)
E1
S1
Write a balanced equation for the half-reaction
that happens at the cathode of this cell.
E2
Of what substance is E1 made?
Write a balanced equation for the half-reaction
that happens at the anode of this cell.
Of what substance is E2 made?
What are the chemical species in solution S1?
S2
What are the chemical species in solution S2?
0
0,0….
X
2
P?
Ni(s) + PdSO4(aq)
1 NiSO4(aq) + Pd(s)
E1
S1
Write a balanced equation for the half-reaction
that happens at the cathode of this cell.
E2
Of what substance is E1 made?
Write a balanced equation for the half-reaction
that happens at the anode of this cell.
Of what substance is E2 made?
What are the chemical species in solution S1?
S2
What are the chemical species in solution S2?
0
0,0….
X
2
P?







