Transcribed Image Text:The retinoblastoma protein (RB) suppresses human cell division by
arresting cells in the G? phase of the cell cycle and preventing
progression to the next phase. It accomplishes this task by binding
to another protein, E2F, a transcription factor needed for further
progression through the cell cycle. Normal progression through the
cell cycle is accomplished when cyclin-dependent kinases (CDKs)
phosphorylate RB, preventing its binding to E2F.
Many viruses can induce abnormal exit from G, using viral proteins
that bind to RB at a motif at the N-terminal called LXCXE. An
example is the E7 papilloma protein, which causes the excessive
proliferation of cells in warts.
The site at which LXCXE proteins bind is called the pocket domain
and is highly conserved on RB and related proteins in plants and
animals. The configuration of the pocket domain is well established.
Mutant experimental RB proteins are available with alterations in the
conserved amino acids of the pocket domain.
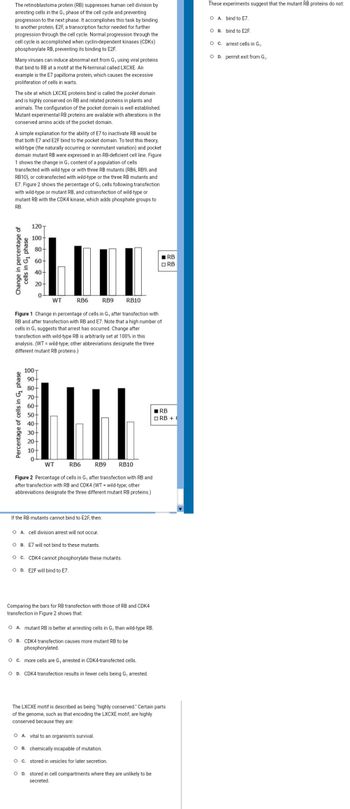
A simple explanation for the ability of E7 to inactivate RB would be
that both E7 and E2F bind to the pocket domain. To test this theory,
wild-type (the naturally occurring or nonmutant variation) and pocket
domain mutant RB were expressed in an RB-deficient cell line. Figure
1 shows the change in G, content of a population of cells
transfected with wild-type or with three RB mutants (RB6, RB9, and
RB10), or cotransfected with wild-type or the three RB mutants and
E7. Figure 2 shows the percentage of G, cells following transfection
with wild-type or mutant RB, and cotransfection of wild-type or
mutant RB with the CDK4 kinase, which adds phosphate groups to
RB.
Change in percentage of
cells in G? phase
120T
100+
80+
60+
40-
20-
0
Percentage of cells in G? phase
WT
RB6
RB9
Figure 1 Change in percentage of cells in G, after transfection with
RB and after transfection with RB and E7. Note that a high number of
cells in G, suggests that arrest has occurred. Change after
transfection with wild-type RB is arbitrarily set at 100% in this
analysis. (WT = wild-type; other abbreviations designate the three
different mutant RB proteins.)
100-
90-
80-
70-
60-
50-
40-
30-
20-
10-
LLLL
RB6
RB9 RB10
0
WT
Figure 2 Percentage of cells in G, after transfection with RB and
after transfection with RB and CDK4 (WT = wild-type; other
abbreviations designate the three different mutant RB proteins.)
If the RB mutants cannot bind to E2F, then:
RB10
O A. cell division arrest will not occur.
O B. E7 will not bind to these mutants.
O C. CDK4 cannot phosphorylate these mutants.
OD. E2F will bind to E7.
Comparing the bars for RB transfection with those of RB and CDK4
transfection in Figure 2 shows that:
RB
ORB
O A. mutant RB is better at arresting cells in G? than wild-type RB.
O B. CDK4 transfection causes more mutant RB to be
phosphorylated.
O c. more cells are G, arrested in CDK4-transfected cells.
O D. CDK4 transfection results in fewer cells being G? arrested.
RB
ORB + (
O A. vital to an organism’s survival.
OB. chemically incapable of mutation.
O c. stored in vesicles for later secretion.
O D. stored in cell compartments where they are unlikely to be
secreted.
The LXCXE motif is described as being “highly conserved.” Certain parts
of the genome, such as that encoding the LXCXE motif, are highly
conserved because they are:
These experiments suggest that the mutant RB proteins do not:
O A. bind to E7.
O B. bind to E2F.
O
C. arrest cells in G?.
OD. permit exit from G?.
arresting cells in the G? phase of the cell cycle and preventing
progression to the next phase. It accomplishes this task by binding
to another protein, E2F, a transcription factor needed for further
progression through the cell cycle. Normal progression through the
cell cycle is accomplished when cyclin-dependent kinases (CDKs)
phosphorylate RB, preventing its binding to E2F.
Many viruses can induce abnormal exit from G, using viral proteins
that bind to RB at a motif at the N-terminal called LXCXE. An
example is the E7 papilloma protein, which causes the excessive
proliferation of cells in warts.
The site at which LXCXE proteins bind is called the pocket domain
and is highly conserved on RB and related proteins in plants and
animals. The configuration of the pocket domain is well established.
Mutant experimental RB proteins are available with alterations in the
conserved amino acids of the pocket domain.
A simple explanation for the ability of E7 to inactivate RB would be
that both E7 and E2F bind to the pocket domain. To test this theory,
wild-type (the naturally occurring or nonmutant variation) and pocket
domain mutant RB were expressed in an RB-deficient cell line. Figure
1 shows the change in G, content of a population of cells
transfected with wild-type or with three RB mutants (RB6, RB9, and
RB10), or cotransfected with wild-type or the three RB mutants and
E7. Figure 2 shows the percentage of G, cells following transfection
with wild-type or mutant RB, and cotransfection of wild-type or
mutant RB with the CDK4 kinase, which adds phosphate groups to
RB.
Change in percentage of
cells in G? phase
120T
100+
80+
60+
40-
20-
0
Percentage of cells in G? phase
WT
RB6
RB9
Figure 1 Change in percentage of cells in G, after transfection with
RB and after transfection with RB and E7. Note that a high number of
cells in G, suggests that arrest has occurred. Change after
transfection with wild-type RB is arbitrarily set at 100% in this
analysis. (WT = wild-type; other abbreviations designate the three
different mutant RB proteins.)
100-
90-
80-
70-
60-
50-
40-
30-
20-
10-
LLLL
RB6
RB9 RB10
0
WT
Figure 2 Percentage of cells in G, after transfection with RB and
after transfection with RB and CDK4 (WT = wild-type; other
abbreviations designate the three different mutant RB proteins.)
If the RB mutants cannot bind to E2F, then:
RB10
O A. cell division arrest will not occur.
O B. E7 will not bind to these mutants.
O C. CDK4 cannot phosphorylate these mutants.
OD. E2F will bind to E7.
Comparing the bars for RB transfection with those of RB and CDK4
transfection in Figure 2 shows that:
RB
ORB
O A. mutant RB is better at arresting cells in G? than wild-type RB.
O B. CDK4 transfection causes more mutant RB to be
phosphorylated.
O c. more cells are G, arrested in CDK4-transfected cells.
O D. CDK4 transfection results in fewer cells being G? arrested.
RB
ORB + (
O A. vital to an organism’s survival.
OB. chemically incapable of mutation.
O c. stored in vesicles for later secretion.
O D. stored in cell compartments where they are unlikely to be
secreted.
The LXCXE motif is described as being “highly conserved.” Certain parts
of the genome, such as that encoding the LXCXE motif, are highly
conserved because they are:
These experiments suggest that the mutant RB proteins do not:
O A. bind to E7.
O B. bind to E2F.
O
C. arrest cells in G?.
OD. permit exit from G?.







